
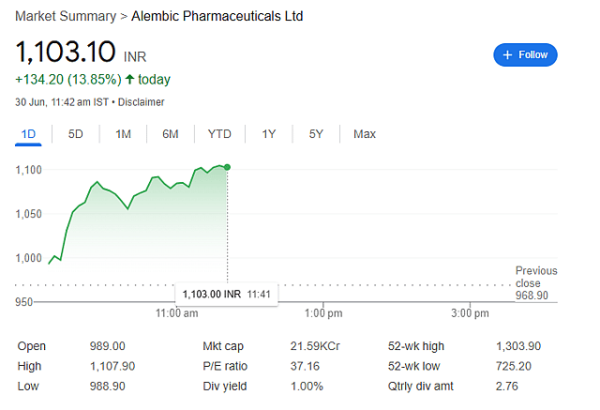
Mumbai: Shares of Alembic Pharmaceuticals surged 13.61 percent on Monday after a major regulatory approval. The stock hit a high of Rs 1,104.10 during the day.
The reason for this rise? Alembic got final approval from the US Food and Drug Administration (USFDA) for its cancer drug — Doxorubicin Hydrochloride Liposome Injection.
About the Approved Drug
This injection is used to treat:
- Ovarian Cancer
- AIDS-related Kaposi’s Sarcoma
- Multiple Myeloma
The drug will be available in two strengths:
20 mg/10 mL (2 mg/mL)
50 mg/25 mL (2 mg/mL)
According to IQVIA, the US market size for this medicine is about $29 million for the 12 months ending March 2025.
Market Outlook 24th Jun 2025: Technical Call Of The Day & Top 5 Stocks In Focus For 24th June 2025Alembic’s Growing USFDA Approvals
With this approval, Alembic now has a total of 224 USFDA-approved drugs:
- 201 final approvals
- 23 tentative approvals
This strengthens the company’s presence in the regulated US market.
Stock Technicals and Valuation
Technically, the stock is in a strong zone and trades above all major moving averages (5-day to 200-day SMAs).
Its 14-day RSI is 74.41, which means the stock is now in an overbought zone.
From a valuation point:
P/E Ratio: 34.36
P/B Ratio: 4.30
EPS: Rs 32.03
Return on Equity (RoE): 12.51 percent
Beta (1-year): 0.46 (low volatility)
Strong Promoter Holding
As of March 2025, promoters hold 69.67 percent stake in Alembic Pharmaceuticals. This indicates strong promoter confidence.
-
Good news for Team India ahead of 2nd IND vs ENG Test, star player ignored due to…

-
Abhishek Bachchan breaks silence on divorce rumours and negative news, says, ‘When you have a…’

-
Dubai announces first successful test flight of air taxi ahead of 2026 rollout

-
Wimbledon chaos as pro-Palestine protesters swarm tennis tournament

-
Wimbledon chaos as pro-Palestine protesters swarm tennis tournament
