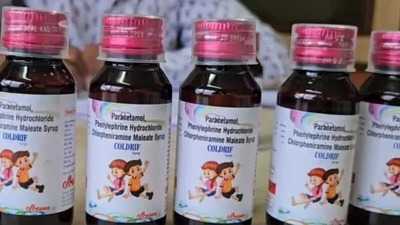
New Delhi: The Delhi government has banned the sale, purchase and distribution of Coldrif cough syrup after it was declared “not of standard quality”, an official order said.
According to the order issued on Friday, Coldrif Syrup (Paracetamol, Phenylephrine Hydrochloride, Chlorpheniramine Maleate Syrup), manufactured in May 2025 by Sresan Pharmaceutical Manufacturer, Tamil Nadu, was found to be adulterated with Diethylene Glycol (46.28 per cent w/v), a toxic chemical known to be harmful to human health.
All stakeholders are directed to immediately stop selling, purchasing, or distributing the said batch of the syrup. The general public has also been advised not to use the product, given its potential health risks, the order said.
The assistance of all stakeholders is sought for the strict implementation and wide dissemination of the public advisory, which has been issued in public interest, it said.
-
Sainik School Admission 2026: Applications for Sainik School Admission Open, Apply Here
-
GATE Exam Last Date: Big news for students! Now apply with late fees until this date.

-
India–EFTA trade pact targets $100 billion in investments, 10 lakh jobs over 15 years

-
Future of India-Afghanistan relations is very bright: Afghan Foreign Minister

-
Lapses by TN Food & Drug department exposed in Coldrif probe